Why do elements bond with each other?
In ionic bonding one element's atom shares its outer layer electrons in order to fill in another element's atom's outer layer.
It looks something like this:
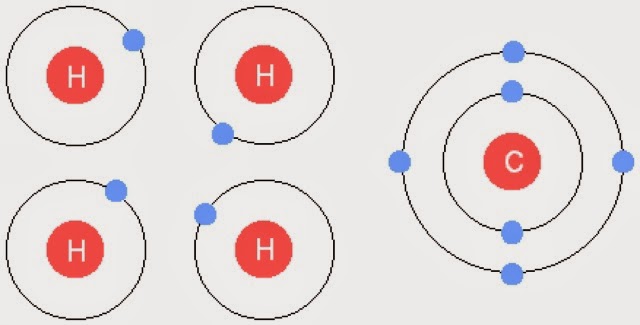
In covalent bonds atoms share there electrons with other atoms. The outer layer electrons fit on both atom's outer layers in order to fill them up.
It looks something like this:
To find out more about bonding and chemistry in general and see these pictures along with other examples go to
http://faculty.clintoncc.suny.edu/faculty/michael.gregory/files/bio%20101/bio%20101%20lectures/chemistry/chemistr.htm